Mevalonate pathway

The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria.[1] The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.[2]
The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP.[3] It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway.
Upper mevalonate pathway
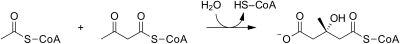
[edit]The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form HMG-CoA (3-hydroxy-3- methyl-glutaryl-CoA). Reduction of HMG-CoA yields (R)-mevalonate. These first 3 enzymatic steps are called the upper mevalonate pathway.[4]
Lower mevalonate pathway
[edit]The lower mevalonate pathway which converts (R)-mevalonate into IPP and DMAPP has 3 variants. In eukaryotes, mevalonate is phosphorylated twice in the 5-OH position, then decarboxylated to yield IPP.[4] In some archaea such as Haloferax volcanii, mevalonate is phosphorylated once in the 5-OH position, decarboxylated to yield isopentenyl phosphate (IP), and finally phosphorylated again to yield IPP (Archaeal Mevalonate Pathway I).[5] A third mevalonate pathway variant found in Thermoplasma acidophilum, phosphorylates mevalonate at the 3-OH position followed by phosphorylation at the 5-OH position. The resulting metabolite, mevalonate-3,5-bisphosphate, is decarboxylated to IP, and finally phosphorylated to yield IPP (Archaeal Mevalonate Pathway II).[6][7]
Regulation and feedback
[edit]Several key enzymes can be activated through DNA transcriptional regulation on activation of SREBP (sterol regulatory element-binding protein-1 and -2). This intracellular sensor detects low cholesterol levels and stimulates endogenous production by the HMG-CoA reductase pathway, as well as increasing lipoprotein uptake by up-regulating the LDL-receptor. Regulation of this pathway is also achieved by controlling the rate of translation of the mRNA, degradation of reductase and phosphorylation.[1]
Pharmacology
[edit]A number of drugs target the mevalonate pathway:
- Statins (used to decrease cholesterol levels);
- Bisphosphonates (used to treat various bone-degenerative diseases such as osteoporosis[8])
Diseases
[edit]A number of diseases affect the mevalonate pathway:
- Mevalonate Kinase Deficiency
- Mevalonic Aciduria
- Hyperimmunoglobulinemia D Syndrome (HIDS).
Alternative pathway
[edit]Plants, most bacteria, and some protozoa such as malaria parasites have the ability to produce isoprenoids using an alternative pathway called the methylerythritol phosphate (MEP) or non-mevalonate pathway.[9] The output of both the mevalonate pathway and the MEP pathway are the same, IPP and DMAPP, however the enzymatic reactions to convert acetyl-CoA into IPP are entirely different. Interaction between the two metabolic pathways can be studied by using 13C-glucose isotopomers.[10] In higher plants, the MEP pathway operates in plastids while the mevalonate pathway operates in the cytosol.[9] Examples of bacteria that contain the MEP pathway include Escherichia coli and pathogens such as Mycobacterium tuberculosis.
Enzymatic reactions
[edit]| Enzyme | Reaction | Description |
| Acetoacetyl-CoA thiolase |  |
Acetyl-CoA (citric acid cycle) undergoes condensation with another acetyl-CoA molecule to form acetoacetyl-CoA |
| HMG-CoA synthase | Acetoacetyl-CoA condenses with another Acetyl-CoA molecule to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). | |
| HMG-CoA reductase |  |
HMG-CoA is reduced to mevalonate by NADPH. This is the rate limiting step in cholesterol synthesis, which is why this enzyme is a good target for pharmaceuticals (statins). |
| mevalonate-5-kinase | 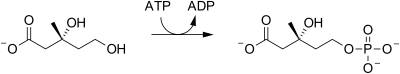 |
Mevalonate is phosphorylated at the 5-OH position to yield mevalonate-5-phosphate (also called phosphomevalonic acid). |
| mevalonate-3-kinase | 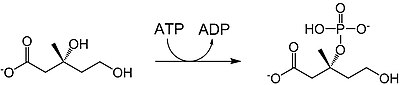 |
Mevalonate is phosphorylated at the 3-OH position to yield mevalonate-3-phosphate. 1 ATP is consumed. |
| mevalonate-3-phosphate-5-kinase |  |
Mevalonate-3-phosphate is phosphorylated at the 5-OH position to yield mevalonate-5-phosphate (also called phosphomevalonic acid). 1 ATP is consumed. |
| phosphomevalonate kinase |  |
mevalonate-5-phosphate is phosphorylated to yield mevalonate-5-pyrophosphate. 1 ATP is consumed. |
| mevalonate-5-pyrophosphate decarboxylase |  |
Mevalonate-5-pyrophosphate is decarboxylated to yield isopentenyl pyrophosphate (IPP). 1 ATP is consumed. |
| isopentenyl pyrophosphate isomerase | 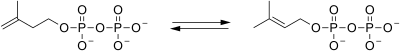 |
isopentenyl pyrophosphate is isomerized to dimethylallyl pyrophosphate. |
References
[edit]- ^ a b Buhaescu I, Izzedine H (2007) Mevalonate pathway: areview of clinical and therapeutical implications. ClinBiochem 40:575–584.
- ^ Holstein, S. A., and Hohl, R. J. (2004) Isoprenoids: Remarkable Diversity of Form and Function. Lipids 39, 293−309
- ^ Goldstein, J. L., and Brown, S. B. (1990) Regulation of the mevalonate pathway. Nature 343, 425−430
- ^ a b Miziorko H (2011) Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch Biochem Biophys 505:131-143.
- ^ Dellas, N., Thomas, S. T., Manning, G., and Noel, J. P. (2013) Discovery of a metabolic alternative to the classical mevalonate pathway. eLife 2, e00672
- ^ Vinokur JM, Korman TP, Cao Z, Bowie JU (2014) Evidence of a novel mevalonate pathway in archaea. Biochemistry 53:4161–4168.
- ^ Azami Y, Hattori A, Nishimura H, Kawaide H, YoshimuraT, Hemmi H (2014) (R)-mevalonate-3-phosphate is an intermediate of the mevalonate pathway in Thermoplasma acidophilum. J Biol Chem 289:15957–15967.
- ^ Lewiecki, E. Michael (May 2010). "Bisphosphonates for the treatment of osteoporosis: insights for clinicians". Therapeutic Advances in Chronic Disease. 1 (3): 115–128. doi:10.1177/2040622310374783. ISSN 2040-6223. PMC 3513863. PMID 23251734.
- ^ a b Banerjee A, Sharkey TD. (2014) Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Nat Prod Rep 31:10431055
- ^ Orsi E, Beekwilder J, Peek S, Eggink G, Kengen SW, Weusthuis RA (2020). "Metabolic flux ratio analysis by parallel 13C labeling of isoprenoid biosynthesis in Rhodobacter sphaeroides". Metabolic Engineering. 57: 228–238. doi:10.1016/j.ymben.2019.12.004. PMID 31843486.
External links
[edit]- Rensselaer Polytechnic Institute page on cholesterol synthesis (including regulation)